News
News Articles 31 - 40 of 160
Jan
Read more
2021 was a year of significant progress for the IQILS accreditation programme. The programme is in its strongest position since it launched back in 2021. The first few onsite accreditation assessments were conducted in the latter stages of 2021. We would like to congratulate the services at University Hospital Southampton NHS Foundation Trust and North Bristol NHS Trust on being awarded accreditation. Dr James Ferguson, the IQILS clinical lead has commented on the outstanding level of care, compassion and innovation these services have demonstrated. Several assessments will be taking place in the early part of 2022. Currently the programme has 61 registered services, 2 accredited services and 12 services are currently signed off for the level 1 standards.
Introduction to IQILS accreditation programme – Tuesday 15 February 2022, 09:30 – 11:00
If you would like to learn more about the IQILS programme. A virtual introduction to IQILS accreditation session is being ran. This session is designed for services not yet registered to the programme and are interested in finding out more. During the session delegates will learn about:
• What is accreditation and how it can add value to your service
• The process and logistics involved in accreditation
• The overall benefits of registering
The session will be led by the Dr James Ferguson, IQILS clinical lead and Dr Andrew Yeoman, the QA lead of the IQILS programme. The session will take place via MS teams. Once you have registered an invite will be sent to all delegates with the dial in details. Registration for services is open.
Services registered to the programme receive access to the following benefits:
• The full accreditation standards
• Access to the IQILS website and self-assessment webtool
• Regular virtual training session on the accreditation process and standards
• Online repository of best practice templates and documents
• Regular newsletters on the programme with updates
• Support from the IQILS office team
If your service is interested in registering, please visit the IQILS website to sign up; https://www.iqils.org/UserRegistration/ .
Accreditation helps services to:
• independently measure themselves against national standards
• reduce variation in quality standards of their services
• demonstrate dedication to improvement, patient safety and reducing risk
• raise their profile, which can often be used to leverage support in their organisation
• highlight good practice and get targeted advice on where to focus improvement efforts
Please contact the IQILS team at askiqils@rcp.ac.uk or 0203 3075 1508 if you would like to speak to the team about the programme. IQILS has recently joined twitter, follow us @IQILS_Liver .
(Added 5/01/2022)
Dec
Read more
With the rapid rise in cases of the Omicron variant of COVID-19 in the UK the NHS potentially faces another surge in hospital admissions and we are all facing familiar uncertainty and drawing on our resilience mechanisms again. The uncertainty is compounded by not yet knowing the severity or otherwise compared to the Delta variant, the moderating effect of vaccination and whether enough of the population are either vaccinated or boosted to exert this effect, and how the new monoclonal antibody drugs for symptomatic mild to moderate disease in the community will impact on admissions. Below is a summary from the relevant policies, with links, for your information.
These new monocolonals now have conditional marketing authorisation for use in non-hospitalised patients in the UK (read the cas.mhra.gov.uk website alert by clicking > here ).
- Sotrovimab is given by single intravenous infusion and is reported to reduce the relative risk of hospitalisation or death by 85%.
- Molnupiravir is given orally by capsules for 5 days and is reported to reduce the relative risk of hospitalisation or death by 30%.
Patients must meet all of the eligibility criteria and none of the exclusion criteria. Prehospitalised patients are eligible for treatment if:
• SARS-CoV-2 infection is confirmed by polymerase chain reaction (PCR) testing within the last 5 days
AND
• Onset of symptoms of COVID-193 4 within the last 5 days
AND
• A member of a ‘highest’ risk group (as defined in Appendix 1).
Symptoms listed are: feverish, chills, sore throat, cough, shortness of breath or difficulty breathing, nausea, vomiting, diarrhoea, headache, red or watery eyes, body aches, loss of taste or smell, fatigue, loss of appetite, confusion, dizziness, pressure or tight chest, chest pain, stomach ache, rash, sneezing, sputum or phlegm, runny nose.
Appendix 1 includes liver disease in the following categories:
• Patients with cirrhosis Child’s-Pugh class B and C (decompensated liver disease).
• Patients with a liver transplant
• Liver patients on immune suppressive therapy (including patients with and without liver cirrhosis)
• Patients with cirrhosis Child’s-Pugh class A who are not on immune suppressive therapy (compensated liver disease)
Patients will be anxious, and as clinicians we may be contacted for advice. How the delivery of these drugs is to be organised is being worked out at local operational levels. Members are advised to make enquiries within their Trusts for local arrangements. The Liverpool COVID-19 interactions checker is a useful resource (click > here ). We checked these drugs against the more commonly used immunosuppressants and, for those not familiar with this tool, it is easy to use (no expected interactions for tacrolimus, ciclosprin, azathioprine, mycophenolate, prednisolone).
Members will no doubt be aware that casirivumab and imdevimab (Ronapreve®) has authorisation in hospitalised patients admitted with acute COVID-19. This seems to be less effective in patients with the Omicron variant. Thus where Omicron accounts for more than 50% of the local hospital prevalence, Ronapreve is only recommended where genotyping results are available and confirm infection with a non-Omicron variant. Where genotyping is not available or genotyping confirms infection with the Omicron variant, the December 2021 rapid policy statement advises nMABs can only be offered as part of a formal trial.
Nosocomial acquisition is a bit different. Ronapreve can be used in non-Omicron cases where known, meeting high risk criteria and Sotrovimab has also now been authorised for hospitalised patients who acquire Omicron variant COVID-19 during their hospital stay and meet high risk criteria - for our patients with liver disease, these are the same as those listed above. (read the cas.mhra.gov.uk website alert by clicking > here ).
Again, members are advised to check their local Trust policies, but nosocomial acquisition of COVID-19 in patients with liver disease may well meet criteria for the use of these drugs so we thought we would draw your attention to this.
Hepatologists and gastroenterologists have faced very challenging changes to practice over the last two years and rising numbers of patients with liver disease. We know the changing Covid-19 landscape continues to bring new challenges. We hope you all stay safe and well during this period.
Rebecca Jones, President BASL
John Dillon, Vice President Hepatology, BSG
Download Joint BASL_BSG message re monoclonal antibody drugs vs COVID-19.pdf
(Added December 20th 2021)
Nov
Read more
First Call for Applications
The Foundation for Liver Research has set up a new Small Grants Scheme in memory of the late Professor Roger Williams CBE (1931-2020). This scheme is open to any applicant in the UK.
Outline of the Small Grants Scheme
An Application form can be downloaded from the Foundation for Liver Research website > here .
The closing date for the first Call is 1st March 2022.
For details of eligibility and the application process, contact Natalie Day, Chief Executive of the Foundation for Liver Research n.day@researchinliver.org.uk .
Nov
Read more
BASL members are invited to take part in a national survey.
As part of the Early Detection of Liver Disease SIG, we are asking if you could take a moment to complete this short survey of attitudes towards fibrosis assessment and use of non-invasive fibrosis tests.
Gathering this type of information is important to gauge what is current practice and where barriers lie to the use of these tests.
The survey takes only 2 mins, and we’d be hugely grateful for your participation and opinions!
Link to survey: here
Kind Regards,
Kush Abeysekera
Ankur Srivastava
Clinical Research Fellow
Consultant Gastroenterologist and Hepatologist
University of Bristol
Southmead Hospital, North Bristol Trust
Sep
Read more
A survey of HCC surveillance in the UK was last conducted in 2014, which highlighted some weaknesses in service provision nationally. We are repeating the survey to see if things have changed, and extending the survey to include additional questions on surveillance imaging and secondary surveillance. We have worked with the HCC UK Committee in developing this survey, and the project is supported by BASL. We hope that this will generate a valuable contemporary overview of UK HCC surveillance service delivery, and stimulate discussion and innovation.
The link to the survey is found here > https://forms.gle/U46TZq7oeVfJveAs7
This time we are seeking service level responses (one response per organisation or service).We expect the survey will only take 10-15 minutes to complete, although some of the questions might require input from other clinical colleagues e.g. section 3 is about imaging and may require input from a radiologist. We will acknowledge the lead contributor from each organisation in a report of this survey.
Many thanks in advance for your time.
Dr Aloysious Aravinthan, Consultant Hepatologist, Nottingham University Hospitals NHS Trust
Dr James Franklin, Consultant Radiologist, University Hospitals Dorset NHS Foundation Trust
Dr Christopher Clarke, Consultant Radiologist, Nottingham University Hospitals NHS Trust
Professor Steve Ryder, Consultant Hepatologist, Nottingham University Hospitals NHS Trust
Professor Shahid Khan, Professor of Practice (Hepatology), Imperial College London
Aug
Read more
The IQILS accreditation programme has grown significantly in the last 18 months. There has been a rapid increase in the level of engagement from services, committed to progressing with IQILS accreditation. The programme currently has 60 registered services, an increase of 8 services in 2021 alone. 11 services are currently signed off for the level 1 standards; A list of all these services can be found on the IQILS website. Three services will be undergoing a full accreditation assessment in the latter part of 2021.
The programme is also offering registered services, that are not an operational delivery hub for the Hepatitis C network, peer review support opportunities. The peer reviews are taking place remotely and will provide your service with an opportunity to receive feedback and guidance on how to progress with the accreditation standards.
In other news, Dragana Smith has recently been appointed as the new IQILS programme manager. Dragana previously worked as programme manager for the pulmonary rehabilitation accreditation programme, and prior to joining the Royal College of Physicians, worked in the fields of legal practice and medical education.
Registration to the programme is open to all liver services across the UK. If your service is interested in registering, please visit our website to sign up: https://www.iqils.org/UserRegistration/
Services registered to the programme receive access to the following benefits:
• The full accreditation standards
• Access to the IQILS website and self-assessment webtool
• Regular virtual training session on the accreditation process and standards
• Online repository of best practice templates and documents
• Regular newsletters on the programme with updates
• Support from the IQILS office team
Please contact the IQILS team at askiqils@rcp.ac.uk or 0203 3075 1508 if you would like to speak to the IQILS team about the programme.
Aug
Read more
The IQILS accreditation programme is seeking nurse and medical assessors to conduct accreditation assessments for the programme.
The IQILS programme was launched in 2017 and is designed to support liver services in the UK. Accreditation is a supportive process of evaluating the quality of clinical services against the programme’s established standards.
Successful applicants will undergo a detailed training programme that equips them with the necessary skills and knowledge required to undertake the role. You will be invited to remote training sessions, as well as observe an assessment and receive support from the office team. If you are interested in becoming an assessor, please contact the IQILS team at askiqils@rcp.ac.uk to further discuss the role.
Jun
Read more
Environmentally Sustainable Hepatology - It’s time to make a start.
The Covid-19 Pandemic has precipitated a rapid re-think of how medical services should be provided in the UK and around the world. This reconfiguring of services is happening in parallel to the run up to the G7 conference in Carbis Bay, Cornwall where Climate is a key item on the agenda. As well as this the UK is hosting the COP26 climate conference later this year, this pivotal meeting taking place at a time when key decisions and investments are being made as part of the recovery from the pandemic. There have been many pleas to place Climate, Planetary and Human Health at the centre of the pandemic recovery.
Over the last few years the majority of us will have noticed gradual steps towards a more sustainable future, in what we eat, how we travel and almost every other aspect of our lives. These changes include a move to a more environmentally sustainable future for healthcare. The NHS is leading this change internationally with plans underway to deliver the world’s first net zero healthcare system [1]. This transformation requires a rethink of every aspect of NHS practice from heating and lighting to changes to the NHS constitution. This transformation delivers the potential to maintain or improve quality of care whilst lessening our impact on the planetary systems and natural world on which we all rely.
"It is not enough for the NHS to treat the problems caused by air pollution and climate change - from asthma to heart attacks and strokes - we need to play our part in tackling them at source."
NHS Chief Executive Simon Stevens
A number of specialities including Anaesthetics, Surgery and Nephrology are making progress with a move to more sustainable healthcare models, with help from organisation such as https://sustainablehealthcare.org.uk . Our Gastroenterology colleagues are also making progress with the recent appointment of a Fellow in Sustainable Endoscopy and an expanding international coalition: ‘The Green Endoscopy Network’ (Twitter @GreenEndoscopy).
It is overdue for Hepatology to start a journey towards a more sustainable future rather than be left on the sidelines at this time.
What is Sustainable Healthcare?
A sustainable healthcare system is achieved by delivery high-quality care and improved public health without exhausting natural resources and living within the ecological boundaries of our planet.
The 4 main domains of sustainable healthcare are set out below adapted from Mortimer et al.
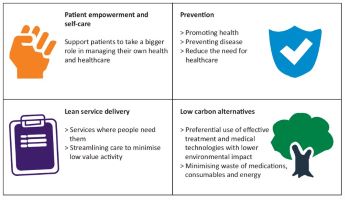
How do we make a start on Sustainable Hepatology?
Maybe the best way to start is by recognising that some current practices already fit within the sphere of sustainable hepatology within one of the domains above. For instance non-invasive assessment of hepatic fibrosis whether it be by ELF test or FibroScan represent low carbon modalities of assessing severity of liver disease. Other more sustainable practices underway, such as telephone and video appointments where appropriate, represent a change to lean services delivery and such practices should be embedded where appropriate and feasible. These may sound like small steps, however with an increasing burden of liver disease they are significant and represent a beginning.
Where else might Sustainable Hepatology lead and what might be the next steps?
The next step may be to consider embedding sustainability in quality improvement framework (SusQI) and into the outcomes of a service measured against environmental, economic, and social cost to determine its sustainability value. This framework was developed by the Centre for Sustainable Healthcare with partners including the Royal College of Physicians [2]. This might lead to a new focus on healthcare preventive measures such as minimum unit pricing of alcohol or a greater focus on preventing metabolic liver disease both within childhood and adult services.
We might also consider:
• Form a multi-disciplinary group of interested others to define what Sustainable Hepatology should be.
• Consulting with https://sustainablehealthcare.org.uk at an early stage.
• Educate ourselves, our association, colleagues, departments and others where appropriate on climate, health and liver disease.
• Incorporate talks on climate and sustainability into gastroenterology and hepatology training and relevant meetings.
• The principles of sustainable quality improvement (SusQI) have been developed by a group including the Royal College of Physicians. These principles could be incorporated into guideline development and quality improvement in Hepatology.
• Appoint Fellows in Sustainable Hepatology (as is taking place in Endoscopy, Anaesthetics, Surgery, Dentistry and Psychiatry).
• A move to more sustainable Hepatology conferences.
In our opinion it is time for hepatology to look to a more sustainable future and we welcome the thoughts and opinions of colleagues in the UK around the world as to how to make this happen.
Join BASL’s Sustainable Hepatology Working Group
BASL is committed to being an inclusive organisation, offering equal opportunity for members to be involved in the work of the association. If you are interested in being part of a working group to develop ideas for Sustainable Hepatology and work with others to promote thinking about this is our future research and service developments, please contact our lead, Dr William Stableforth, by emailing william.stableforth@nhs.net .
References
1. https://www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2020/10/delivering-a-net-zero-national-health-service.pdf
2. https://sustainablehealthcare.org.uk/susqi
May
Read more
Each year BASL presents the Dame Sheila Sherlock research prize, one of the highlights of the BASL Annual Meeting. This prize is awarded annually to recognise the enormous contribution of Dame Sheila Sherlock to the development of Hepatology as a discipline in its own right.
Dame Sheila was involved in the foundation of the British Liver Club in 1961, which subsequently evolved into The British Association for the Study of the Liver (BASL). She was one of our past presidents and the first recipient of The BASL Distinguished Service Award. In keeping with Dame Sheila’s enthusiasm for fostering young researchers, this eponymous research prize is awarded to young investigators without substantive posts in either medicine or science for their research contributions in the field of Hepatology.
Eligibility
In keeping with Dame Sheila’s enthusiasm for fostering young researchers, this eponymous research prize is awarded to young investigators without substantive posts in either medicine or science for their contributions in the field of hepatology research.
The winner will receive free registration to the meeting, an award and a prize of £1,000 and an invite to deliver the prize lecture and present their research at the BASL Annual Meeting.
To apply, please send one A4 sheet outlining your research and another A4 sheet listing up to 5 related publications.
Please send submissions to steve@basl.org.uk before the deadline of 09:00hrs on Monday 2nd August 2021
You must be a BASL member to apply.
May
Read more
The Andy Burroughs Young Investigator Award was set up in honour of the late Professor Andrew Burroughs.
Professor Burroughs was an eminent and world renowned Professor of Hepatology and Consultant Physician/Hepatologist and among his many achievements including his wide area of expertise in cirrhosis and portal hypertension and significant contribution to liver Transplantation.
Eligibility
This prize is awarded to young investigators who have contributed to clinical or translational research related to liver disorders who are in training or within 2 years of taking up consultant positions (or equivalent); this year the winner will receive free registration to the meeting, an award and a prize of £1,000 and an invite to deliver the prize lecture and present their research at the BASL Annual Meeting.
To apply, please send one A4 sheet outlining the research and another A4 sheet listing up to 5 related publications.
Please send submissions to steve@basl.org.uk before the deadline of 09:00hrs on Monday 2nd August 2021
You must be a BASL member to apply.

